About HOME
Summary
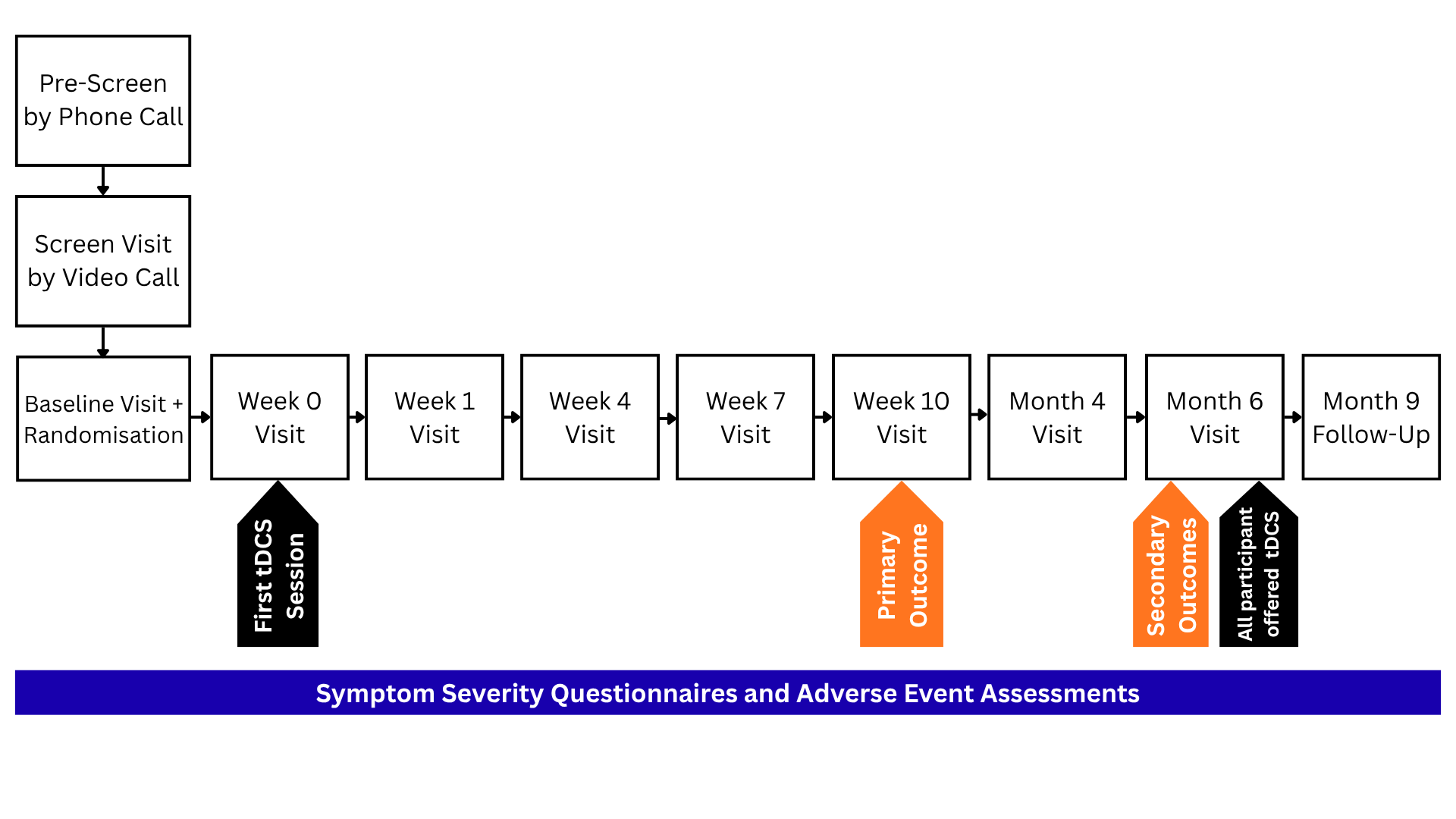
HOME is a multi-centre, two-parallel group, superiority randomised controlled trial to evaluate home-based transcranial direct current stimulation (tDCS) in major depressive disorder.
The primary outcome is to evaluate clinical effectiveness at 10-week end of treatment between the two treatment arms (treatment as usual (TAU) and TAU plus tDCS) as the difference in clinician-rated depressive symptom severity.
The study is sponsored by King’s College London and co-sponsored by South London and Maudsley NHS Foundation Trust.
The study is funded by the National Institute for Health and Care Research (NIHR) (REF: NIHR165425). The Leeds East Research Ethics Committee has provided ethics approval (IRAS ID: 344318).
Trial Information
REC Reference: 25/YH/0166
IRAS: 344318
ISRCTN: https://www.isrctn.com/ISRCTN14516822
Sponsor: King’s College London
Co-Sponsor: South London and Maudsley NHS Foundation Trust
Funder: National Institute for Health and Care Research (NIHR)
Enrolment: 438
Study Population
Inclusion Criteria
Adults aged 18 years or over
Having major depressive disorder (MDD) based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria (APA, 2013) assessed by structured clinical assessment, Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998)
Having at least a moderate severity of depressive symptoms, as measured by a rating score of 18 or more in Montgomery-Asberg Rating Scale (MADRS)
Either not taking antidepressant medication or taking a stable dose of antidepressant medication for at least 6 weeks before enrolment.
Either not currently in psychotherapy or in ongoing psychotherapy for at least 6 weeks before enrolment
Being under the care of GP
Agreeable for GP to be regularly informed about study participation
Able to provide written, informed consent
Exclusion Criteria
Having sgnificant suicide risk as measured by Columbia Suicide Severity Rating Scale (C-SSRS) Screen (Posner et al., 2011)
Having a primary comorbid psychiatric disorder based on DSM-5 criteria as assessed in MINI
Current daily use of medications that affect cortical excitability (e.g. benzodiazepines)
Current illicit drug use or heavy alcohol use with high risk of alcohol use disorder as measured by Alcohol use disorders identification test consumption (AUDIT C) (Khadjesari et al., 2017; NICE, 2023)
History of electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), cranial electrotherapy stimulation (CES), transcranial direct current stimulation (tDCS), deep brain stimulation (DBS), or other brain stimulation
Having history of esketamine / ketamine for treatment of depression
Having history of psychosurgery for depression
Having cognitive impairment (e.g. dementia)
Having a current medical disorder or neurological disorder that may mimic mood disorder
Having any implant in the brain or neurocranial defect
Having shrapnel or any ferromagnetic material in the head
Having any active implantable medical device (e.g. pacemaker)
If female and of child-bearing potential, currently pregnant or planning to become pregnant during the study
Concurrent enrolment in another research treatment study
Study Arms
Participants will be randomised into one of the two arms: treatment as usual (TAU) or TAU plus tDCS. Participants will be aware of the treatment arm to which they have been allocated.
Primary Objective
To evaluate clinical effectiveness as the difference in depressive symptom severity as measured by the clinician-rated MADRS at the 10-week end of treatment between the two treatment arms: those receiving treatment as usual (TAU) and those receiving TAU plus tDCS.
Secondary Objectives
To evaluate sustained clinical effectiveness as the difference in depressive symptoms as measured by MADRS at the 6-month follow up between the two treatment arms.
To assess further clinical outcomes at 10-week end of treatment and at 6-month follow up.
To evaluate clinical effectiveness at 10-week end of treatment period between treatment arms as the difference in self-rated depressive symptoms
To evaluate clinical effectiveness at 10-week end of treatment period between treatment arms as the difference in clinician-rated depressive symptoms
To evaluate anxiety symptoms at 10-week end of treatment period between treatment arms as the difference in clinician-rated anxiety symptoms
To evaluate treatment response at 10-week end of treatment period between treatment arms
To evaluate treatment remission at 10-week end of treatment period between treatment arms
To evaluate sustained clinical effectiveness at 6 months between treatment arms as the difference in self-rated depressive symptoms
To evaluate sustained clinical effectiveness at 6 months between treatment arms as the difference in clinician-rated depressive symptoms
To evaluate sustained anxiety symptoms at 6 months between treatment arms as the difference in clinician-rated anxiety symptoms
To evaluate sustained treatment response at 6 months between treatment arms
To evaluate sustained treatment remission at 6 months between treatment arms